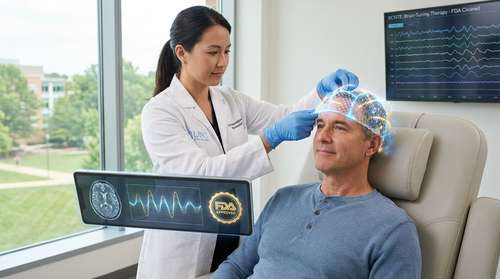
In a historic milestone for mental health treatment, the FDA has cleared ProlivRx, the first prescription, physician-directed at-home neuromodulation device for adults with Major Depressive Disorder (MDD). The clearance, granted to neurotech pioneer Neurolief, introduces a new treatment paradigm for the millions of Americans battling treatment-resistant depression. As of this week, clinicians are preparing to integrate this groundbreaking technology into practice, marking the beginning of a new era where hospital-grade brain stimulation is accessible from the comfort of a patient's living room.
Breaking the Clinic Barrier: What is ProlivRx?
ProlivRx represents a significant departure from traditional depression treatments. Unlike oral antidepressants, which can cause systemic side effects, or Transcranial Magnetic Stimulation (TMS), which requires daily clinic visits, ProlivRx is designed for at-home use under remote physician supervision. The headset utilizes a proprietary technology called eCOT-AS (external Combined Occipital and Trigeminal Afferent Stimulation), which delivers precise, gentle electrical pulses to the occipital and trigeminal nerves—key pathways involved in mood regulation.
"Until now, patients struggling with difficult-to-treat depression did not have a non-pharmacological therapy option that could be administered at home," stated Dr. Linda Carpenter, co-lead investigator of the MOOD clinical trial and Director of the Neuromodulation Program at Butler Hospital. "ProlivRx bridges the gap between medication and invasive procedures, offering a powerful tool for those who have failed to find relief with standard antidepressants."
MOOD Clinical Trial: The Data Behind the Approval
The FDA's decision was supported by the compelling results of the MOOD clinical trial, a multicenter, randomized, sham-controlled study conducted across the U.S. and Israel. The trial evaluated the device's safety and efficacy in patients with MDD who had not responded adequately to at least one antidepressant medication. Key findings released in the approval package include:
- Higher Remission Rates: In the 8-week double-blind phase, 21.3% of patients using ProlivRx achieved full remission, compared to just 6.0% in the sham (placebo) group.
- Sustained Improvement: During the open-label phase extending to 16 weeks, remission rates climbed to 32%, with patients experiencing a mean reduction of nearly 10 points on the Hamilton Depression Rating Scale (HDRS17).
- Safety Profile: The treatment was well-tolerated, with the most common adverse event being mild, transient headaches. No systemic side effects were reported, a major advantage over pharmacotherapy.
Physician-Directed, Patient-Centered
While ProlivRx is self-administered at home, it remains a physician-directed therapy. The device is connected to a cloud-based digital platform that allows psychiatrists to monitor patient adherence and progress in real-time. This "digital leash" ensures that patients receive the same level of oversight they would in a clinic, without the logistical burden of daily travel. Scott Drees, CEO of Neurolief, emphasized this benefit during the rollout announcement: "We are democratizing access to advanced neuromodulation. This is about meeting the patient where they are—both physically and clinically."
A New Hope for Treatment-Resistant Depression
The approval comes at a critical time. An estimated one-third of MDD patients do not respond to initial antidepressant treatments, leaving a massive population with limited options. Traditional alternatives like TMS or ECT (electroconvulsive therapy) are effective but resource-intensive and often inaccessible to those in rural areas. ProlivRx's clearance creates a scalable solution that can be deployed rapidly across health systems.
BrainsWay, a global leader in noninvasive neurostimulation, has already signaled its confidence in the technology through a strategic investment in Neurolief. Industry analysts predict that the U.S. rollout, commencing in early 2026, will likely target integrated care settings first, allowing for a streamlined prescription process for patients currently cycling through ineffective medications.
Availability and Next Steps
As the rollout begins this month, patients are advised to consult their psychiatrists about eligibility. The device is indicated as an adjunctive treatment for adults with MDD who have failed at least one antidepressant. With insurance coverage discussions already underway, ProlivRx is poised to become a cornerstone of modern psychiatric care, offering a drug-free, evidence-based lifeline to those who need it most.