If you have noticed that a pulled muscle or minor injury takes significantly longer to heal in your 60s than it did in your 20s, you are not alone. For decades, scientists believed this slowdown was simply a sign of cellular wear and tear. However, a groundbreaking UCLA aging study 2026 has turned this assumption on its head. Published in the journal Science on January 30, 2026, the research identifies a specific protein that forces aging muscle stem cells to make a critical choice: prioritize their own survival over repairing your tissue.
The 'Cellular Brake': Uncovering the NDRG1 Protein
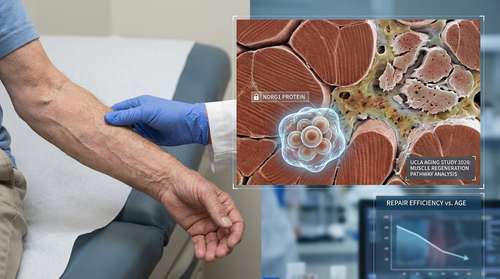
The study, led by Dr. Thomas Rando at the UCLA Broad Stem Cell Research Center, has identified a protein called NDRG1 (N-myc downstream regulated gene 1) as the culprit behind slow healing in older adults. In young muscle tissue, stem cells are primed for action—they are like sprinters in the starting blocks, ready to divide and repair damage the moment injury strikes.
However, as we age, our muscle stem cells accumulate high levels of NDRG1. This protein acts as a "cellular brake," suppressing the mTOR signaling pathway that normally drives cell growth and regeneration. According to the researchers, this isn't a malfunction, but a survival strategy. By slowing down, these stem cells protect themselves from the metabolic stress of replication, allowing them to survive longer in the harsh environment of aging tissue.
The Survivor's Dilemma: Resilience vs. Regeneration
This discovery highlights a biological trade-off that researchers are calling the "Survivor Paradox." The study revealed that while NDRG1 protein aging mechanisms make stem cells more resilient, they drastically reduce muscle regeneration in seniors.
"Think of it like a marathon runner versus a sprinter," explained Dr. Rando in a statement regarding the findings. "The stem cells in young animals are hyper-functioning—really good at what they do, namely sprinting—but they aren't built for the long term. Older cells have adapted to run the marathon of life, prioritizing their own persistence over immediate performance."
Can We Reverse the Process?
To test this theory, the team blocked NDRG1 in aged mice. The results were immediate and striking: the old stem cells "woke up" and began repairing muscle tissue at youthful speeds. However, this rejuvenation came at a heavy cost. Without the protective braking mechanism of NDRG1, the stem cell population depleted rapidly, leaving the muscle with fewer reserves for future repairs. This finding suggests that current sarcopenia treatment breakthroughs must find a delicate balance between boosting repair speed and maintaining the stem cell pool.
Implications for Sarcopenia Treatment Breakthroughs
Sarcopenia, the age-related loss of muscle mass and function, affects millions of older adults and is a leading cause of frailty. This UCLA study suggests that simply "revving up" old cells might not be the silver bullet scientists hoped for, as it could exhaust the body's regenerative reserves. Instead, future therapies may focus on temporarily lifting the NDRG1 brake during critical recovery windows rather than permanently removing it.
This nuanced understanding of muscle stem cell repair opens new doors for targeted drugs that could help seniors recover from surgery or falls without compromising their long-term muscle health.
Healthy Aging Mobility Tips: What You Can Do Now
While pharmaceutical interventions based on NDRG1 are still years away, you can support your muscle health today. Current research into healthy aging mobility tips suggests that lifestyle factors can influence how your muscles age:
- Prioritize Protein: Aging muscles become less efficient at processing protein. Aim for 25-30 grams of high-quality protein per meal to stimulate muscle protein synthesis.
- Resistance Training: While stem cell activity slows, mechanical loading through weightlifting or resistance bands remains the most potent signal for muscle maintenance.
- Anti-Inflammatory Diet: Chronic inflammation accelerates stem cell dysfunction. specific diets rich in omega-3 fatty acids and antioxidants may help preserve the muscle microenvironment.
This landmark UCLA study fundamentally changes how we view aging. It suggests that our bodies aren't just failing as we get older; they are actively making strategic compromises to keep us going. Understanding this "survivorship bias" is the first step toward therapies that don't just make us live longer, but help us heal better.