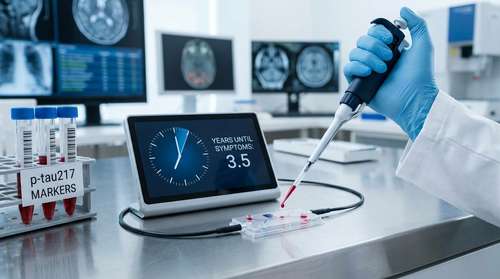
A revolutionary breakthrough in Alzheimer's research has arrived: a simple blood test that functions as a biological "clock," predicting when cognitive symptoms will begin with remarkable accuracy. Researchers at Washington University School of Medicine in St. Louis have developed a model that tracks the accumulation of the specific protein marker p-tau217 to forecast the onset of memory loss within a margin of three to four years. Published on February 19, 2026, in Nature Medicine, this diagnostic tool promises to transform how we approach brain aging, offering a critical window for preventative cognitive health strategies.
How the Alzheimer's Blood Test Clock Works
The core of this innovation lies in its ability to read the body's molecular history. Much like counting the rings on a tree to determine its age, the accumulation of the protein p-tau217 in the blood follows a consistent, predictable pattern. This protein is a specific marker for the neurofibrillary tangles found in the brains of Alzheimer's patients.
Senior author Dr. Suzanne E. Schindler, an associate professor of neurology at WashU Medicine, explains that while amyloid and tau proteins begin building up in the brain decades before symptoms appear, their rate of accumulation in the blood allows for a precise timeline calculation. By measuring plasma p-tau217 levels, the new model can estimate not just if a person will develop dementia, but when those symptoms are likely to manifest.
The "Tree Ring" Effect
The study, led by Kellen K. Petersen, PhD, utilized data from over 600 older adults. The team discovered that once p-tau217 levels reach a specific threshold, the trajectory toward symptoms becomes highly predictable. This "molecular clock" provides a timeline that was previously impossible to determine without expensive and invasive PET scans or spinal fluid tests.
Key Findings: Age Determines Resilience
One of the study's most crucial insights is how age influences the speed of disease progression. The research revealed that younger brains are significantly more resilient to the toxic effects of protein accumulation than older brains. This variance is critical for accurate early dementia prediction.
For example, if a person shows elevated p-tau217 levels at age 60, they may not develop cognitive symptoms for another 20 years. However, if the same elevated levels are first detected at age 80, symptoms typically emerge within just 11 years. This distinction allows clinicians to tailor preventative advice and potential treatments based on a patient's specific life stage, rather than a one-size-fits-all approach.
Revolutionizing Clinical Trials and Preventative Health
Currently, finding participants for clinical trials who are on the verge of developing symptoms is a major bottleneck in drug development. This new brain aging diagnostic test solves that problem by identifying individuals in the "sweet spot" for testing preventative therapies—those who have the pathology but haven't yet suffered memory loss.
By effectively screening participants using a low-cost blood test rather than expensive imaging, researchers can accelerate the development of drugs designed to halt the disease before irreversible damage occurs. The test uses the PrecivityAD2 platform, developed by C2N Diagnostics, a WashU startup, making it a commercially viable option for widespread research use immediately.
The Future of Healthy Aging Breakthroughs
While this tool is currently recommended primarily for clinical trials and research settings, its implications for the general public are profound. As we move through 2026, the shift from reactive treatment to preventative cognitive health is gaining momentum. Knowing one's risk timeline could eventually empower individuals to make lifestyle changes—such as diet modifications, exercise regimens, and vascular health management—years before symptoms would otherwise appear.
"Eventually, the goal is to be able to tell individual patients when they are likely to develop symptoms, which will help them and their doctors to develop a plan to prevent or slow symptoms," Dr. Schindler stated. As validation studies continue, this blood test represents a monumental leap toward a future where Alzheimer's is managed as a chronic, preventable condition rather than an inevitable decline.